Welcome to the blog of the project MetClimVOC!
March 2021
How gas surface interactions can affect VOC monitoring?
Gases in contact with solid surfaces can interact in different ways, depending on the nature of both substances and the thermodynamic conditions. In industry and research, gas - solid interactions are inherent part of the work, especially between the materials used in pipes and devices and the gas of interest.
Interactions between solid surfaces and gas can represent a loss or gain to the species mass balance. Estimating the magnitude of these changes and the characteristic times of interactions helps to improve the reproducibility of measuring systems and the estimation of uncertainty of the measurements.
An important part of the monitoring of VOCs in air is the process of collecting the samples which can be performed off-line or online, depending on the application. In online methods, the sampling process occurs continuously for a specific duration of time and directly feeds the measurement instrument. In off-line sampling methods, samples are first collected in a special container and later analysed in the laboratory.

Figure 1: Offline sampling devices: canisters and Tedlar bags used for sampling air.
Both offline and online sampling processes in environmental applications rely on the use of devices such as canisters, bags and sampling pumps that expose the gas sample to possible changes in composition. For this reason, when estimating the composition of a sample, it is necessary to keep in mind the changes that could have affected the sample from the time of sampling to that of measurement.
# What type of interactions can we expect?
Depending on the morphology of the solid and the conditions of operation different phenomena can occur. The most fundamental types of interactions to take into account are the sorption phenomena: adsorption (capture) and desorption (release). Any fluid- solid system will have a bulk zone where convection and diffusion processes are controllant and a surface layer in direct contact with the solid, where the sorption phenomena occurs. The capture or release of molecules due to sorption is a reversible process. The direction and magnitude of the mass transfer in the sorption process is directly proportional to the local difference in the amount of substance fraction at the surface layer of the solid wall. The rate of adsorbed or desorbed mass will greatly depend on the affinity of the molecules in the gas phase to those of the solid surface.
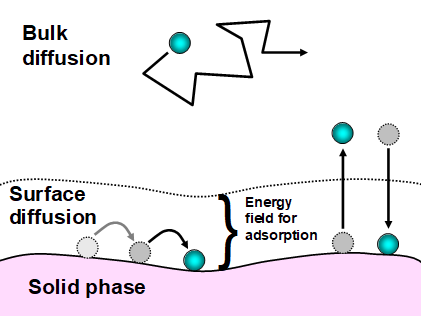
Figure 2: Surface layer phenomena in a fluid-solid system [Hubbe, 2019]
In porous systems, such as polymeric materials (i.e. PFA, PTFE, FEP, PEEK), a process called permeation takes place. Permeation is a combination of both sorption and diffusion, where the VOC molecules diffuse through the solid pores, moving from the zones with higher amount of substance fraction to that with the lowest. Adsorption also occurs inside the pores of the material. Diffusion is a much slower process than adsorption with a rate that depends on the difference in the amount of substance fraction per unit of length and the diffusion coefficient of the compound in the matrix of interest.
If the porous material stays in contact with a VOC gas mixture for enough time, the system will reach an equilibrium where a certain amount of VOC molecules are adsorbed to the pores of the material. If the material is then put into a clean system, it would act like a source of mass, with internal desorption and diffusion occuring inside of the material and desorption occuring from the surface layer to the gas bulk, what is known as memory effect.
Reactions can also occur between oxidising or acidic molecules and the solid material, especially in the presence of water and porous and non - porous system. Depending on the type of material and the compounds involved, reactions can be reversible or irreversible processes with highly variable reaction rates.
# How to know what interactions do I have in my experimental system?
Rather than what, the question should be how to identify your relevant interactions.
An important step to determine which interactions are actually relevant is the evaluation of the characteristic time. Each system have a specific dynamic that is usually delimited by the slower or controlling phenomena occurring. The characteristic time of a system is the time required to reach equilibrium from any state (e.g. relaxation time). The faster the process, the shorter the characteristic time.
To estimate the characteristic time you should start with a few questions. Are your samples staying in the lab for days or hours before the measurement? Or, on the contrary, do you have an on-site measurement system that directly pulls the sample? Try to identify batch (e.g. offline operations) from continuous (e.g. online operations) sections in your processes to understand what could be the critical areas in terms of mass loss.
Having enough information about the dynamics of your process, it is time to understand the dynamics of the actual interactions. A common qualitative method for testing the materials, consists in the observation of the time delay of a system - with a known area of test material – after a sudden change in the amount of substance fraction (step signal) [Deming et al., 2019]. The time required to reach stability is a common way to have an idea of how much time it takes for a specific material to be in equilibrium with the mixture. It is useful because it does not require to understand exactly what phenomena occur in the system. With the knowledge of the delay time we can actually adapt our system to operate under conditions where we guarantee the effects of interactions will not affect the measurements. The main limitation with this method is that it does not account for irreversible interactions and does not quantify losses due to interaction. However, this method is helpful to compare materials: the shorter delay is associated with a higher inertness.
# What about quantification?
There are times when you want to estimate the mass loss due to interactions, e.g. to complete the definition of the uncertainty. A conservative approach for the estimation of acetone loss rate due to adsorption have been proposed by [Sassi et al., 2015]. The method estimates the maximum loss expected based on an equilibrium constant determined experimentally. The figure below shows the loss rate of acetone as function of the area for different materials and amount of substance fractions.
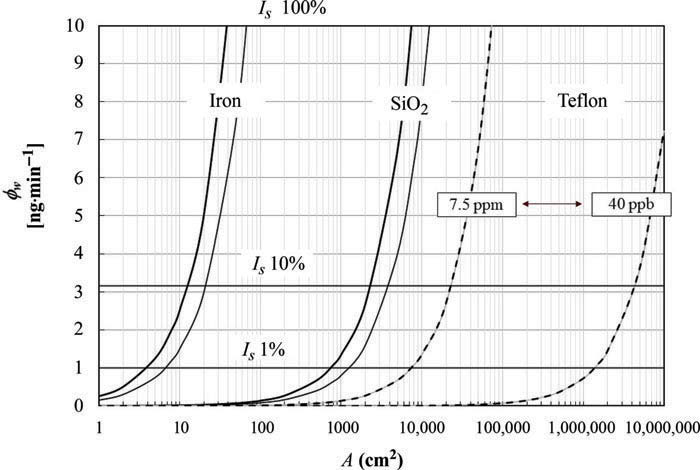
Figure 3: Wall interaction flow rate (φW ) vs. wall area (A) for case study device (Acetone in dry air – reference characteristic time for acetone interaction = 20 min).
Considering that a typical dilutor area can be in the range from 300 to 600 cm2, the expected loss for iron, glass and Teflon can be estimated. The reference time of acetone interaction (time delay) was around 20 min. So measurement and sampling processes overlapping with this characteristic time will have to account for the maximum interaction. Thresholds Is are the limits for negligible, relevant and maximum contribution of adsorption to the uncertainty of the measured acetone concentration of the case study.
The experimental procedure for the determination of the equilibrium constant and the influence of operating conditions has been recently published [Sassi et al, 2021]. The reproducibility of the method was studied with tests over a Sulfinert® / Acetone case study, and it was observed to be better than 2.5%. In the case of losses due to reactions or irreversible interactions, the procedure would have to be integrated by means of a steady state mass balance.
# How does MetClimVOC contribute?
In MetClimVOC project we aim to improve the understanding of the effects that sampling lines have in the uncertainty of VOC measurements at trace level. POLITO, IMTelecom, UU and IL will evaluate the effect of different sampling lines in the measurements of several challenging oxy-VOCs and terpenes, with amount of substance fractions at ambient levels. Moreover, we will investigate the effects of humidity, sampling temperature and flow rates in the monitoring while testing porous and non porous materials that are commonly used in gas metrology. These results will improve the definition of the uncertainty of both oxy-VOCs and terpenes measurements, that will now include the effects of reversible, irreversible and permeation interactions.
#About the authors

Maricarmen Lecuna, Politecnico di Torino. Chemical Engineer with background of process design, working in Metrology since 2014. She is currently Post-Doc in POLITO’s “Metrology for Process Engineering” research group, involved in the EMRP projects KEY-VOCs and MetAMC and the EMPIR project MetAMCII.
#Latest posts
Go back to the latest blog post.
# Other posts
In this section, you can find the links to other blog posts.
February 2022 - Reference gas mixtures for oxy-VOCs
December 2021 - Terpenes in the atmosphere
October 2021 - Monitoring halogenated VOCs from the space
June 2021 - One year of project MetClimVOC!
January 2021 - Halogenated VOCs in the atmosphere: monitoring and traceability
November 2020 - Atmospheric trace gases: VOCs, impact climate change
September 2020 - Welcome to the blog of the project MetClimVOC!